Project: Transcranial direct current Stimulation for Focal Refractory epilepsy in Mitochondrial disease (TRANSFORM)
Based at: Wellcome Centre for Mitochondrial Research, Newcastle
Research Leads: Dr Mark Baker, Prof Robert McFarland
In a nutshell, what is the idea behind TRANSFORM?
TRANSFORM is the name of a research study into a possible way of treating epilepsy in children and young adults with mitochondrial disease. Epileptic seizures can be a prominent and debilitating symptom of mitochondrial diseases, especially in children. These seizures are often ongoing and very difficult to control.
Currently the only way to manage the seizures is with multiple medications, each of which may have unpleasant side effects, or in severe cases to admit the patient to intensive care for general anaesthesia with all the complications that entails. Often medication or hospitalisation can’t fully control the seizures anyway, so there’s a real need for an alternative treatment.
How does the treatment work?
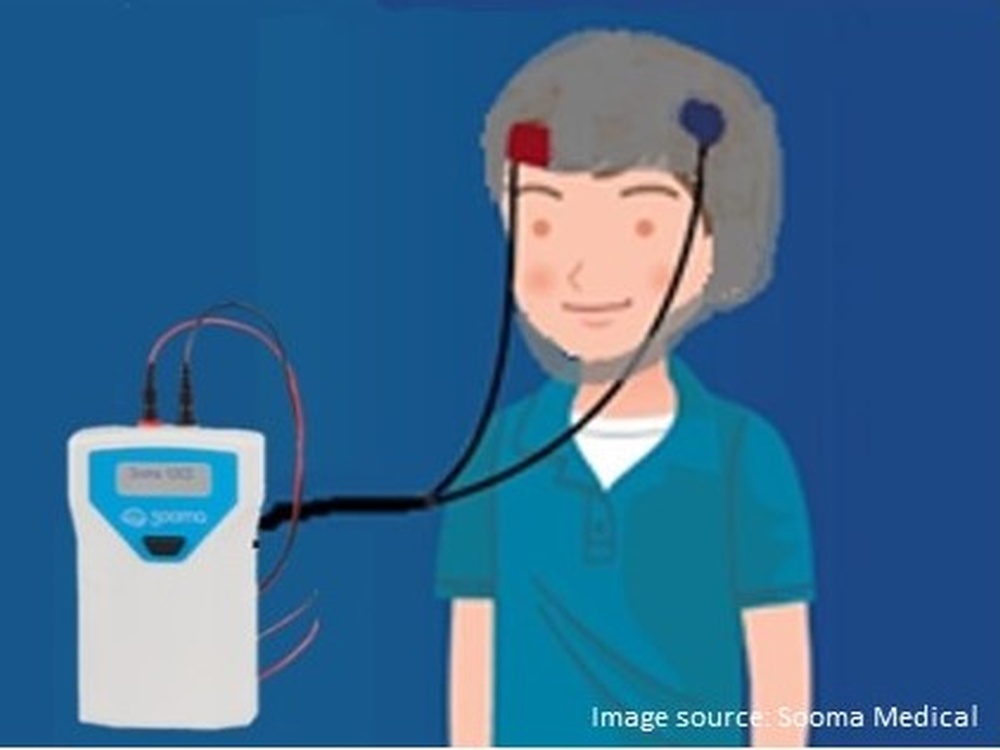
Seizures are the result of abnormal electrical activity in the brain. The therapy used in this study is called transcranial direct current stimulation (tDCS) and involves the use of a device that delivers a tiny electrical current to the brain in order to calm the electrical activity causing the seizures.
It’s essentially a little box with electrodes that connect to a cap; the cap is designed specifically for each individual patient based on whereabouts in the brain their seizures are determined to be taking place. The treatment is given for 20 minutes daily for a maximum of 14 days and could even be applied at home. In fact, devices like these are already being successfully used in other areas of medicine such as depression and pain management.
What stage is the project at now?
The project was hampered by the COVID-19 pandemic but now things are moving apace and we’re looking for more participants to join the study. Our first patient successfully took part in the trial, which proved that our study plan works well. After very careful preparation and a training session, the parents were able to use the device on their own child, and we’re delighted to report that their feedback was very positive.
The device was deemed to be quick and easy to use, and there were no negative side-effects, which gives us confidence that the treatment is safe to use more widely. However, we’re talking about a very rare disease here, and this is making it really challenging for us to find enough study participants within the limited time we’re running the study.
Who can participate?
To be considered for the trial, patients must be aged two years or over, have a genetically proven mitochondrial disease diagnosis and suffer from drug-resistant focal epilepsy. Ideally we would like all potential patients to attend a screening visit up here in Newcastle to determine suitability for the study.
We need to measure the brainwaves through an EEG or MRI to confirm the patient is in fact suffering from focal seizures i.e. seizures that happen in only one area of the brain, as that’s the condition where we think the treatment helps. However if that process has already taken place at a different hospital, or the patient is simply unable to travel, there are always workarounds!
What evidence is there that the treatment can work?
Research studies have shown that tDCS is effective to treat epilepsy in other diseases. We’d already offered a few mitochondrial disease patients this treatment and their seizures stopped shortly after. Then we designed the study to measure the effectiveness of tDCS for mitochondrial epilepsy in a systematic way. It was challenging because we need a control group to make sure our study effects are true effects caused by the treatment rather than the placebo. However, we didn’t want to withhold the treatment from any study participant who may benefit from it. I believe in the end we found a good compromise.
Everyone will be ‘blind’ to the two study conditions to avoid any bias. However this does mean that until the end of the study we’re unable to tell how well the treatment works. But in the small number of patients we’ve already offered it to, it’s shown good results. Hence the requirement for more mitochondrial disease patients to now take part.
What are your hopes for the study now?
Since we’ve now successfully tested our first patients, our hope is to involve more patients and spread the word about the study. Hopefully we can treat more people this way and prove the technology works, so that eventually it can become a regular, recognised treatment for mitochondrial disease symptoms that any consultant can offer. This would be an achievable, accessible option for patients that far outweighs more drastic, dangerous alternatives. And as well as becoming a mainstream treatment for mitochondrial disease patients with epilepsy, we have hope that it could even be expanded to help manage epilepsy in other conditions too.
How has The Lily Foundation contributed to this research?
The funding provided by The Lily Foundation has helped make this research possible, so we’re extremely grateful for their confidence in our concept. Their grant allowed us to invest in commercial, user-friendly tDCS devices – much easier for us and of course for the patients!
The Foundation are still closely involved now, spreading the word about the study on social media, playing a vital role in the recruitment of new mitochondrial disease patients to the study, and checking in every few months to see how things are developing. TRANSFORM is something that is very close to our hearts, and we’d like to explore this as a treatment for one of the most significant symptoms of mitochondrial disease in children and young adults.
When might this treatment be available to patients?
It’s so hard to put a timeline on things, but it’s likely to be a few years yet before clinicians could offer tDCS at their practice as a standard treatment outside of research. We were hampered so much by the pandemic, and now that we’re able to move ahead with the study we desperately need more patients to come forward.
If we recruit enough participants, we would plan to complete all the data collection by the end of 2024. But only after we’ve tested the final patient and completed the study can we then analyse the results and understand whether it’s been a success. There are not many treatments available for patients with mitochondrial disease and epilepsy, so it’s a really exciting project, and one that we are determined to see through to the end.
Study note:
The TRANSFORM research study is now open to people with a mitochondrial disease diagnosis and drug-resistant focal epilepsy.
If you would like to participate, or know somebody who fits the criteria and would be interested in taking part, please contact us: Prof Robert McFarland at +44 191 2825225, Dr Albert Lim ([email protected]), Katrin Bangel ([email protected]) or visit the study summary page.