
Questions & answers
Do you have a question about mitochondrial disease? You’ll find the answer here.

Do you have a question about mitochondrial disease? You’ll find the answer here.
Whether you’ve recently received a diagnosis or are researching on behalf of a loved one, we hope these frequently asked questions can provide some clarity and ease your journey with mitochondrial disease.
This Q&A section has been carefully crafted by The Lily Foundation and reviewed by our expert medical board. It offers general advice to help improve understanding of mitochondrial disease and is for educational purposes only. The Lily Foundation does not warrant that the information we provide will meet your health or medical requirements. It does not constitute medical advice and the charity is not liable should it be treated as medical advice.
Every case of mitochondrial disease is unique, so if you have concerns about your health or that of your child, you should consult a specialist or your NHS clinician directly.
For more on mitochondrial terminology, check out our mitochondrial disease glossary.
Mitochondria are tiny structures found in almost every cell in our bodies. They convert the food we eat into the energy we need to survive and grow.
Mitochondria are tiny and contain only 37 genes.
The number of mitochondria in each cell can vary between one and many thousand, depending on how much energy that cell requires. Busy cells like those in the brain, heart or liver need a lot of energy to function properly and therefore have lots of mitochondria.
Inside these mitochondria, a chain of chemical reactions takes place that changes the food we’ve eaten and digested into a form of energy that cells in our body can use. This process is known as oxidative phosphorylation, and it takes place in the respiratory chain.
Scientists split this process into five separate reactions, known as Complexes. Complex I to IV involve the transfer of electrons along a pathway, and Complex V completes the process, with the end result being production of energy that our bodies can use. There are a number of complex chemical changes that happen during this production line involving hundreds of proteins, each with its own special job to do.
Mitochondrial disease is actually an umbrella term used to describe a vast group of rare inherited conditions affecting energy production.
Our mitochondria perform many tasks but mitochondrial disease is the result of problems converting energy within the mitochondria.
Without the right amount of energy, our cells cannot do their job and they start to die. If a lot of mitochondria in the body are affected, especially in important body organs, a mitochondrial disorder can be very serious and often fatal.
Each affected individual will have different mitochondrial disease symptoms and severity because a different combination of their mitochondria are working and not working within each cell, and each person might also have different cells in the body affected.
The underlying genetic cause may be different for different people, but all will result in the inability of the mitochondria to produce the right amount of energy.
There are many different types of mitochondrial diseases, with more being discovered through research all the time. Some types of the disease affect only one organ, and some affect multiple organs.
There are times when particular organs or body systems are affected in a recognisable pattern and these have been given ‘syndrome names’. Examples of these types of mitochondrial diseases include Alper’s, Leigh syndrome, MELAS or MERRF, to name just a few.
Mitochondrial dysfunction might also be classified by the specific component of oxidative phosphorylation that it affects e.g. Complex I, or by the specific gene mutation that has caused it.
Take a closer look at our What is mitochondrial disease? page to find out more.
You can also learn more about the different types of mitochondrial disease syndromes.
Mitochondrial disease is a rare disease.
It’s believed that 1 in 200 babies in the UK are born with genetic changes that could cause a mitochondrial disorder, although not all of these will go on to develop serious disease.
The incidence of mitochondrial dysfunction in the adult population is estimated at around 1 in 4,300 (Gorman et al. Annals of Neurology 2015). As we learn more about mitochondrial disease, and as diagnostic tools improve, we believe we’re likely to see an increase in diagnosis rates.
However true numbers are very hard to determine due to the wide range and varying severity of symptoms experienced, which means it’s often overlooked or misdiagnosed. In addition, the range of genetic mutations that can cause the disease is vast.
The severity of mitochondrial disease ultimately depends on the underlying genetic cause. With maternally inherited mitochondrial disease, a person may have a certain percentage of their mitochondria not working.
If it’s a small percentage, they may not develop symptoms, their symptoms may be mild or not begin until later in life. If it’s a large percentage then symptoms often begin in early childhood and can be severe and often life limiting, shortening the patient’s lifespan.
Certain types of mitochondrial syndromes can present with more severe symptoms than others, and there are also circumstances where the severity is organ-dependent, for example the brain in Leigh syndrome – the involvement of such a key organ makes this a very severe form of mitochondrial disease with knock-on effects to many other organs.
The various numbers refer to the various processes involved in energy production (oxidative phosphorylation) in the mitochondria. A higher number is not worse – it just helps doctors explain which part of the mitochondria might not be working properly and helps direct treatment or genetic testing.
Because these mitochondrial components live alongside each other, if one portion isn’t working properly, another portion may also not work as intended, so a person can have more than one complex affected.
These different stages in the process are referred to as Complex I, Complex II, Complex III, Complex IV and Complex V. When any one of these steps is blocked, usually because a genetic defect has prevented the manufacture of a protein required for that step, mitochondrial disease can occur.
When doctors are diagnosing a patient with mitochondrial disease, tests may indicate which part of this process is not working properly and the patient might be described as having a particular complex affected eg. ‘Complex I Mitochondrial Disease’ indicates that the problem has arisen in the first stage (the first complex) of the respiratory chain.
For a long time, mitochondrial disease was considered a rare childhood disorder, however in the last decade it’s been discovered that many more common disorders, including Parkinson’s disease and Alzheimer’s disease, may have some evidence of mitochondrial dysfunction.
In addition, some forms of cancer, diabetes and epilepsy can result from problems with mitochondrial function.
So despite being a little-known condition, mitochondrial disease could be the key to some of the most important medical breakthroughs of our time.
You can’t catch mitochondrial disease; you’re born with it, and the way it’s inherited is extremely complicated.
Depending on the underlying genetic error, mitochondrial disease can be inherited from the mother or father or both. It’s also possible that the error may have arisen for the first time in the affected person. This is particularly hard to understand if mitochondrial disease has arisen in a child from two apparently healthy adults.
The error is usually the result of a genetic defect (a mutation in our genes) which disrupts energy production. This can present at birth or later in life. This defect can be in either our mitochondrial DNA (mtDNA) or in our nuclear DNA.
You can find out more about mitochondrial disease inheritance on the NHS Rare Mitochondrial Disorders website.
It is. Many people don’t realise that in fact we all have two different types of DNA in our bodies.
Most people are familiar with nuclear DNA, which looks like a twisting ladder and makes up 99.9% of the total DNA in our bodies. It’s responsible for determining all the unique characteristics that make us who we are.
But less well-known is mitochondrial DNA (mtDNA) which is a tiny ring-shaped structure. Unlike nuclear DNA, our mitochondrial DNA plays no part in determining our unique characteristics but is crucial for the production of our energy.
Nuclear DNA
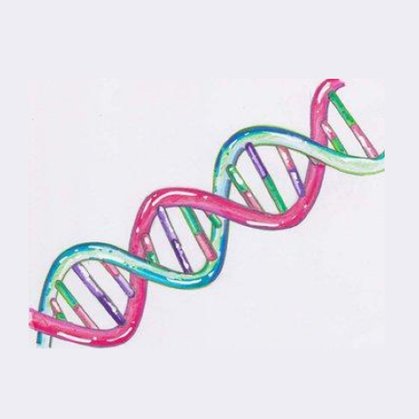
Nuclear DNA is found within the nucleus of our cells. It’s linear in shape and makes up 99.9% of the total DNA in our body.
Within the cell, the nuclear DNA is packaged up into structures called chromosomes. Humans have 46 chromosomes per cell which are arranged in pairs (23 of these come from your mother and 23 from your father). These chromosomes contain between 20,000-25,000 genes.
Nuclear DNA (and the genes that it holds) are responsible for providing the basis for how human bodies are built and work, as well as determining all our characteristics.
Mitochondrial DNA
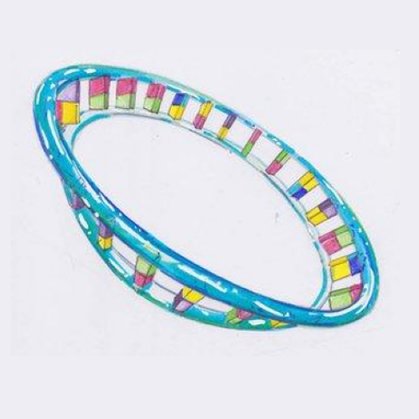
This is a special circular type of DNA that is only found within the mitochondria themselves and it makes up only 0.1% of the total DNA in our bodies.
Mitochondrial DNA contains only 37 genes, all of which are essential for normal mitochondrial function (all are essential for oxidative phosphorylation).
Mitochondrial DNA is inherited only from your mother (as the father’s mtDNA is destroyed during fertilisation). Humans contain between 100-1000 copies of mitochondrial DNA per cell.
If you’ve already had a child affected by mitochondrial disease then you’re potentially at risk of this happening again. If you have a genetic diagnosis for your mitochondrial disease, there are options to test future pregnancies, which you will be able to discuss with your consultant.
There are a number of reproductive choices available that are currently able to prevent the transmission of nuclear mitochondrial DNA disease to a child. These include adoption, egg or sperm donation or IVF with Pre-implantation Genetic Diagnosis (PGD). You can find out more about all these techniques on the NHS Rare Mitochondrial Disorders Service website.
In 2015 a new technique was introduced in the UK called Mitochondrial Donation. This is an IVF technique which uses the mother’s and father’s nuclear DNA combined with healthy mitochondria from a donor woman. It prevents the transmission of maternally inherited mitochondrial disease from mother to child and also ensures the child will not carry the disease to its future generations.
For families without a genetic diagnosis, making the decision to have another child involves a degree of risk. The level of risk involved can be estimated from the pattern of inheritance of the disease in the family and is something that would need to be discussed with a doctor.
Mitochondrial disease can cause a variety of different symptoms affecting many different organs and at any age.
Symptoms can vary hugely depending on the actual mitochondrial syndrome or underlying genetic mutation that has caused it.
Children with mitochondrial disease often present with slower than usual physical development, loss of motor skills, damage to the brain, seizures, swallowing problems and breathing difficulties like apnoea (long pauses in breathing pattern).
Adults affected by mitochondrial disease often develop a more slowly progressive illness with, for example, hearing loss, muscle weakness, diabetes, digestive disorders and fatigue.
Mitochondrial disease patients may also suffer from a condition known as lactic acidoisis, which is an increase in lactic acid concentration in the body. This can cause nausea and vomiting, fatigue, fast, deep breathing and muscle cramps.
No, the disease can affect adults as well as children, but it often has different characteristics depending on the age of onset.
Childhood mitochondrial disease is usually more severe due to the nature of the mutations that have been inherited and is often life-limiting.
With adults, mitochondrial disease symptoms may accumulate over time and may be masked by other more common diseases of ageing.
In terms of population prevalence, adult-onset mitochondrial disease is actually much more common than mitochondrial disease in children, partly because childhood-onset disease is often fatal at a very early age whereas adults can be known to survive for many years after a mitochondrial disease diagnosis is made.
Yes – the majority of mitochondrial disease affect boys and girls equally.
However, there is a very small sub-group of mitochondrial diseases that may have a more serious onset in boys than girls because of the way they are inherited. These are known as x-linked disorders and include Pyruvate Dehydrogenase Deficiency (PDHD).
It’s believed that mitochondrial disease affects all ethnic groups equally, although we are not sure that any specific research has been done in this area.
On paper, the incidence may appear higher in the developed world, but this is likely to be due to more advanced healthcare and diagnostic tests available and better record keeping.
The diagnosis of mitochondrial disease often involves a long and complex journey for both patient and doctor. Once a doctor suspects mito disease based on symptoms of the patient, there are a number of tests that must be undertaken to try and confirm the diagnosis.
Blood tests, urine tests, spinal fluid tests and MRI brain scans are often the first tests performed. With the recent advances in genetics, blood samples are often then used to determine if there is a defect in one of the genes known to be an important cause of mitochondrial disease.
Lactate levels are often assessed in routine blood tests when investigating mitochondrial diseases. Whilst lactate measurements can provide valuable insights into mitochondrial issues, they cannot be used on their own to diagnose and monitor the disease. This is because high lactate levels can sometimes be the result of exercise or other non-related conditions.
Mitochondrial disease is very rare. With symptoms of the disease beginning at any age and affecting any body system in ways that are not clearly linked, you can see why it’s particularly challenging to diagnose.
Many mitochondrial disease symptoms are common to other conditions and these need to be ruled out before the diagnosis of a mitochondrial disorder is arrived at.
In addition, there are lots of different genes involved in healthy mitochondrial function, any one of which could go wrong and cause disease, so finding the underlying error through genetic testing is key, but also time consuming.
Mitochondrial disease is a progressive condition which means that the prognosis is for it to get worse over time. A substantial number of children with mitochondrial disorders have a limited lifespan and do not reach adulthood.
The rate of progression does however vary hugely from patient to patient and is dependent on a number of factors including the underlying genetic defect, the organs affected by disease and exposure to infection/medical or physiological stress.
Some mitochondrial disease patients have chronic symptoms that remain stable for a number of years and others may have a slow and steady or rapid downhill progression. However many patients will eventually develop involvement of several organs, and when there is cardiac or brain involvement or stroke-like episodes the disease tends to be more severe.
Every patient is different when it comes to mitochondrial disease.
This topic is still being studied and at present there’s no evidence to link mitochondrial dysfunction with increased rate of infection or indeed immunodeficiency.
We know that having mitochondrial disease leads to more chronic non-life-threatening infections such as colds and ear infections, and in some patients, suffering from these more frequently leads to a decrease in quality of life. This is more likely to be because shrugging off these types of infections might be more difficult for someone with mitochondrial disease, probably as a result of the impact on their other organs.
In 2020, as an initiative of the International Mito Patients (IMP), a group of experts in mitochondrial disease carried out an international study into the safety of medicines in mitochondrial disease patients. The study resulted in the publication of a list of drugs to be avoided or used with caution in primary mitochondrial disease. This list replaces all previous versions and is based on the latest scientific and clinical evidence.
You can find the list and more information on our Drug information for mitochondrial disease page.
If you have a mitochondrial disease diagnosis and are concerned about the medications you’re currently taking, you should discuss this with your doctor at the earliest opportunity.
There’s no evidence that immunisations themselves hurt mitochondria or mitochondrial disease patients. Doctors strongly recommend that patients receive immunisations since infections can produce life-threatening symptoms in patients with mitochondrial disease.
Even though mitochondrial disease is currently incurable, taking certain actions may be helpful in reducing the impact of the condition and limiting further disability, especially as some affected individuals appear more sensitive to external triggers such as minor illness, dehydration, temperature extremes, fatigue and stress.
Following a healthy, balanced diet and keeping well hydrated at all times may help to improve wellbeing. It’s important for mito patients to eat regularly, and to avoid fasting.
Making lifestyle changes, such as taking moderate exercise, could actually improve mitochondrial health. Patients should avoid excessive exposure to toxins such as alcohol or cigarettes. Where possible they should also protect themselves from infectious diseases and manage levels of fatigue or stress sensibly.
Supplements like cofactors (made by the body) or vitamins (from food) may be helpful in slowing the progression of mitochondrial disease. Supplements are often prescribed by clinicians in combination as a ‘cocktail’ tailored to the individual patient.
It’s wise to ensure that someone living with mitochondrial disease has their condition actively monitored in order to identify any changes early, so regular appointments including physical assessments and blood tests are recommended.
Supportive therapies such as physiotherapy, occupational, visual, speech and respiratory therapies can all be beneficial to mitochondrial disease patients.
Although there is currently no cure, there is now money being invested in research into mitochondrial diseases and many exciting discoveries are being published that we hope will lead to treatments to slow the progression of these disorders for future cases.
A new wave of experimental drugs is currently in development, which aim to prevent or reverse some of the main symptoms of mitochondrial disease.
Our commitment to finding a cure for mitochondrial disease is unswerving, and since 2007 we have invested over £3 million in research to develop new treatments. Sign up to our newsletters to be kept up to date with the latest developments in research and breakthroughs in clinical trials.
There are a number of different ways that mitochondrial disease patients and their families can be part of research, but whether you take part in active trials or share your experiences on our IMPACT panel very much depends on what you want to get out of it.
Some studies involve using existing clinical data from routine follow-ups and others require patient participation.
Some trials anonymise data (so you will not get direct personal feedback) and others will provide feedback to participants – either way, your involvement will add huge value to the understanding of this area of medicine. Some studies may not use medical data at all but instead may examine the social impact of this condition.
What exactly will be required from you, and what will be reported back, will depend on the exact nature of the study you sign up to, so it is important to talk to your trial coordinator or clinician to understand what is involved.
Being part of our virtual IMPACT patient committee enables you to help shape future research by getting involved right at the start of the process. You won’t be taking part in medical trials, but you will be helping to shape future research priorities to ensure ongoing work is relevant, impactful and of value to the mitochondrial disease community.
Our understanding of mitochondrial biology has improved along with drug design, and a number of compounds are on the horizon. Huge progress has been made in preventing the transmission of mitochondrial disease and similar advances in therapy are a top priority for researchers throughout the world.
We are also in a better position to assess the efficacy of potential cures and drugs to treat mitochondrial disease as a result of developing patient cohorts and because of highly organised and structured clinical trials.
There is hope.
If you have any problems please email [email protected]